US company withdraws ALS drug after it fails in trial

Amylyx Pharmaceuticals announced Thursday it was withdrawing its approved treatment against the deadly neurodegenerative disease ALS after clinical data found no evidence the drug worked.
In a statement, the US company said it would discontinue its market authorizations for Relyvrio/Albrioza, using the brand names of the medicine in the US and Canadian markets.
"While this is a difficult moment for the ALS community, we reached this path forward in partnership with the stakeholders who will be impacted and in line with our steadfast commitment to people living with ALS and other neurodegenerative diseases," said the company's co-CEOs Joshua Cohen and Justin Klee in a statement.
The company also said it was reducing its workforce "by approximately 70 percent" as it focused on another experimental drug for use against ALS, and on repurposing Relyvrio for other conditions. It added it would continue to make Relyvrio available for patients who wish to keep using the treatment, through a "free drug program."
The news follows data from a clinical trial of 664 ALS patients announced in March, which found no significant differences in outcomes between those on the treatment group and those who received a placebo.
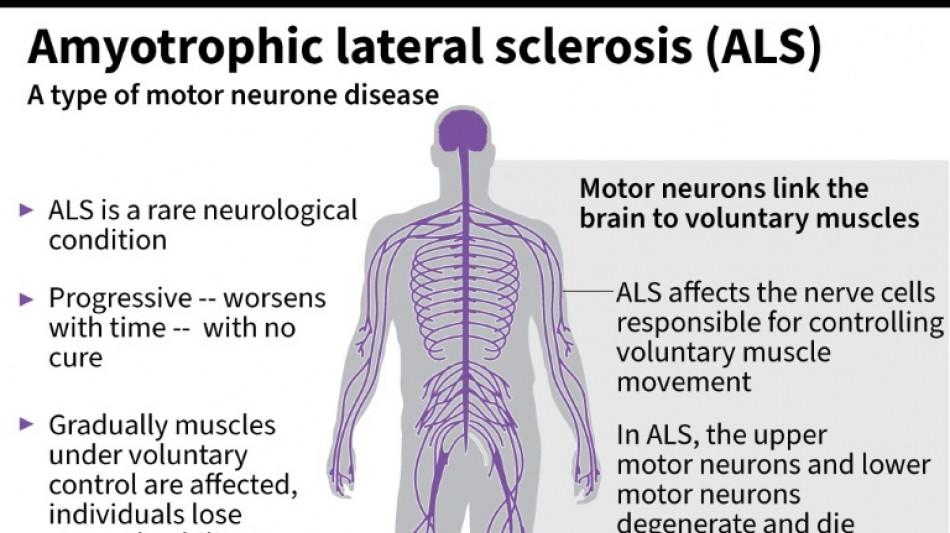
It was a big blow for patients with amyotrophic lateral sclerosis, sometimes called Lou Gehrig's disease after the famous baseball player, which devastates nerve cells in the brain and spinal cord.
ALS affects about two people per 100,000 every year, causing progressive loss of motor and cognitive function. Most patients die within five years of their diagnosis.
Relyvrio's approval by the US Food and Drug Administration in 2022 was controversial and based on the results of a single trial that involved just 137 participants.
The FDA itself noted there was "residual uncertainty about the evidence of effectiveness" -- but "given the serious and life-threatening nature of ALS and the substantial unmet need, this level of uncertainty is acceptable in this instance and consideration of these results in the context of regulatory flexibility is appropriate."
- Patient groups backed approval -
Advocacy groups also mounted a major campaign sending a petition to the FDA with tens of thousands of signatures urging approval. Once it became available, Amylyx reportedly announced an eye-watering list price of $158,000 per year in the US, drawing criticism.
Patient groups in Europe watched with desperation at the bureaucratic delays.
When the European Union drug watchdog later announced it was rejecting Relyvrio, the decision was slammed as "an affront" by angry French patients, who say they "don't have time to wait." France later relented, offering conditional approval in November.
"We commend Amylyx for pulling Relyvrio off the market, while still ensuring that people living with ALS can access the drug if they believe it is helping them," said the US-based ALS association, which had lobbied for the drug's approval and funded its research.
"Safe and potentially effective treatments can be made accessible rapidly until further research can confirm their efficacy," it added.
For now, there remain only a handful of treatments available.
Riluzole, FDA approved in 1995, prolongs life approximately three months. Edaravone, FDA approved in 2017, has been found to slow disease progression and improve survival.
And in 2023, the regulatory body approved tofersen, a gene therapy treatment that targets those ALS cases that are caused by mutations in the SOD1 gene.
(W.Walker--TAG)

 London
London

 Manchester
Manchester
 Glasgow
Glasgow
 Dublin
Dublin
 Belfast
Belfast
 Washington
Washington
 Denver
Denver
 Atlanta
Atlanta
 Dallas
Dallas
 Houston Texas
Houston Texas
 New Orleans
New Orleans
 El Paso
El Paso
 Phoenix
Phoenix
 Los Angeles
Los Angeles